Authors (6): H. A. Bunzel, J. L. R. Anderson, D. Hilvert, V. L. Arcus, M. W. van der Kamp, A. J. Mulholland
Themes: Biocatalysis DOI: 10.1038/s41557-021-00763-6
Citations: 114
Pub type: journal-article
Pub year: 2021
Publisher: Springer Science and Business Media LLC
Issue: 10
License: [{"start"=>{"date-parts"=>[[2021, 8, 19]], "date-time"=>"2021-08-19T00:00:00Z", "timestamp"=>1629331200000}, "content-version"=>"tdm", "delay-in-days"=>0, "URL"=>"https://www.springer.com/tdm"}, {"start"=>{"date-parts"=>[[2021, 8, 19]], "date-time"=>"2021-08-19T00:00:00Z", "timestamp"=>1629331200000}, "content-version"=>"vor", "delay-in-days"=>0, "URL"=>"https://www.springer.com/tdm"}]
Publication date(s): 2021/10/19 (online)
Pages: 1017-1022
Volume: 13 Issue: 10
Journal: Nature Chemistry
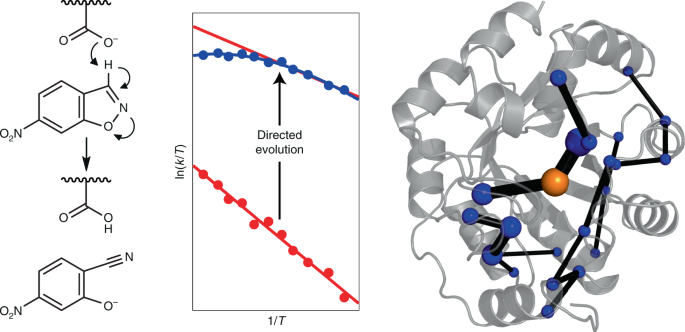
URL: http://dx.doi.org/10.1038/s41557-021-00763-6Activation heat capacity is emerging as a crucial factor in enzyme thermoadaptation, as shown by the non-Arrhenius behaviour of many natural enzymes. However, its physical origin and relationship to the evolution of catalytic activity remain uncertain. Here we show that directed evolution of a computationally designed Kemp eliminase reshapes protein dynamics, which gives rise to an activation heat capacity absent in the original design. These changes buttress transition-state stabilization. Extensive molecular dynamics simulations show that evolution results in the closure of solvent-exposed loops and a better packing of the active site. Remarkably, this gives rise to a correlated dynamical network that involves the transition state and large parts of the protein. This network tightens the transition-state ensemble, which induces a negative activation heat capacity and non-linearity in the activity–temperature dependence. Our results have implications for understanding enzyme evolution and suggest that selectively targeting the conformational dynamics of the transition-state ensemble by design and evolution will expedite the creation of novel enzymes. Computationally designed enzymes can be substantially improved by directed evolution. Now, it has been shown that evolution can introduce a dynamic network that selectively tightens the transition-state ensemble, giving rise to a negative activation heat capacity. Targeting such transition state conformational dynamics may expedite de novo enzyme creation.
| Name | Description | Publised |
|---|---|---|
| 41557_2021_763_Fig4_ESM.webp | Related Article: Bunzel, H. Adrian, Anderson, J. L. Ross, Hilvert, Donal... | 2021 |
| 41557_2021_763_MOESM1_ESM.pdf | Related Article: Bunzel, H. Adrian, Anderson, J. L. Ross, Hilvert, Donal... | 2021 |
| 41557_2021_763_MOESM2_ESM.docx | Related Article: Bunzel, H. Adrian, Anderson, J. L. Ross, Hilvert, Donal... | 2021 |
| 41557_2021_763_MOESM3_ESM.docx | Related Article: Bunzel, H. Adrian, Anderson, J. L. Ross, Hilvert, Donal... | 2021 |
| Evolution of dynamical networks enhances catalysis in a designer enzyme | Data related to: "Evolution of dynamical networks enhances catalysis in ... | 2021 |
<< Previous Back Next >>