Authors (4): S. K. Matam, S. A. F. Nastase, A. J. Logsdail, C. R. A. Catlow
Themes: Core, Featured DOI: 10.1039/d0sc01924k
Citations: 36
Pub type: article-journal
Pub year: 2020
Publisher: Royal Society of Chemistry (RSC)
Issue: 26
License: {"URL"=>"http://creativecommons.org/licenses/by/3.0/", "start"=>{"date-parts"=>[[2020, 6, 17]], "date-time"=>"2020-06-17T00:00:00Z", "timestamp"=>1592352000000}, "delay-in-days"=>168, "content-version"=>"vor"}
Publication date(s): 2020/07/08 (print) 2020 (online)
Pages: 6805-6814
Volume: 11 Issue: 26
Journal: Chemical Science
URL: http://dx.doi.org/10.1039/d0sc01924k
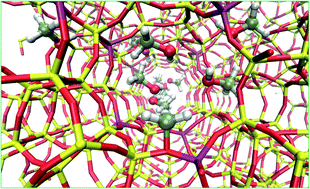
Room temperature methoxylation is methanol loading dependent: the higher the methanol loading, the faster the methoxylation. Methanol load of ≥2 leads to methoxylation while no methoxylation is observed with ≤1 molecule per Brønsted acidic site.
| Name | Description | Publised |
|---|---|---|
| d0sc01924k1.pdf | Supl. data for Methanol loading dependent methoxylation in zeolite H-ZSM... | 2020 |
<< Previous Back Next >>